 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow is carbon monoxide prepared?
Preparation: It is prepared in a number of ways:
1. Pure carbon monoxide can be prepared in the laboratory by heating formic acid with concentrated H2SO4 at 370–4010 K.

2. By burning carbon in a limited supply of oxygen.
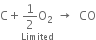
3. By heating carbon dioxide with coke.
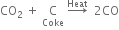
4. By the reduction of oxides of metal with coke.
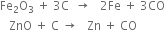
 Short Answer Type
Short Answer TypeDescribe what happens (give chemical equations only) when:
(i) carbon monoxide is treated with chlorine.
(ii) carbon monoxide is passed through heated NaOH under pressure.
(iii) Vapours of carbon monoxide are passed over nickel and
(iv) carbon monoxide is passed through heated ferric oxide.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat is the action of heat on:
(i) Sodium bicarbonate
(ii) calcium carbonate
(iii) Zinc carbonate?
Give one method for industrial preparation and one for laboratory preparation of CO and CO2 each.
