 Short Answer Type
Short Answer TypeExplain the following phenomenon by means of balanced equations:
(i) When exhaling is made through a tube passing into a solution of lime water, solution becomes turgid.
(ii) The turbidity of the above solution.
(iii) eventually disappears when continued exhaling is made through it.
 Long Answer Type
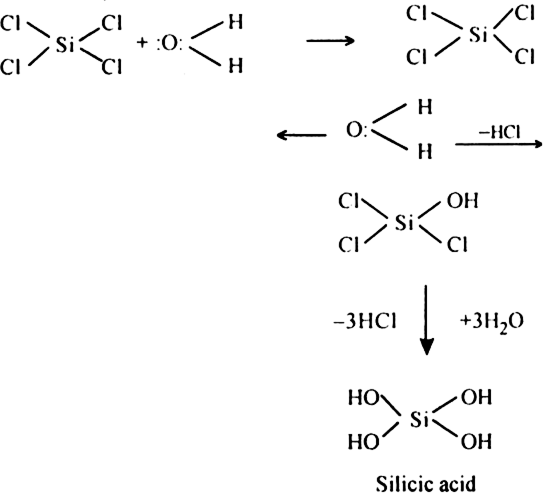
Long Answer TypeExplain why silicon tetrachloride is hydrolyzed but carbon tetrachloride is not hydrolysed.
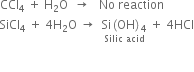
 Short Answer Type
Short Answer Type