 Multiple Choice Questions
Multiple Choice QuestionsAqueous solution of ferric chloride is acidic due to
ionization
polarization
dissociation
hydrolysis
D.
hydrolysis
FeCl3 is the salt of strong acid and weak base, hence hydrolysis of FeCl3 gives acidic aqueous solution.
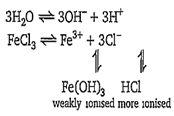
In such cases water becomes acidic because H+ concentration becomes more than OH- concentration. It is also known as cationic hydrolysis.
The correct order of heat of formation of halogen acids is
HI > HBr > HCl > HF
HF > HCl > HBr > HI
HCl > HF > HBr > HI
HCl > HBr > HF > HI
Precipitate of AgCl is soluble in liquid NH3 the compound forms
Ag(NH4)2OH
Ag(NH4)2Cl
Ag(NH3)2OH
Ag(NH3)2Cl
In qualitative analysis, in III group NH4Cl is added before NH4OH because
to increase the concentration of NH ions
to increase the concentration of Cl- ions
to reduce the concentration of OH- ions
to increase the concentration of OH- ions
