 Short Answer Type
Short Answer TypeName the radio active element in group 1. How does it resemble with the remaining elements of the group?
Find out the oxidation state of sodium in Na2O2.
Oxidation state of Oxygen Na2O2 is =(-1)
Let x be the oxidation state of Na in Na2O2. Since peroxide linkage is present in Na2O2 in which the oxidation of O is –1.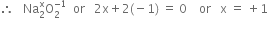
Hence oxidation state of Na in 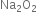 is +1.
is +1.
