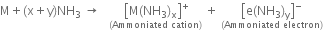Short Answer Type
Short Answer TypeThe ionic radii of alkali metal ions in aqueous solution follows the order Li+ > Na+> K+ > Rb+ > Cs+ Justify the above order.
 Long Answer Type
Long Answer TypeDiscuss the general trends in ionisation enthalpy and electropositive character of alkali metals.
 Short Answer Type
Short Answer TypeLithium ion has the lowest and caesium ion has the highest mobility in an electric field. Explain.
When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reactions for this type of colour change.
Or
Explain why alkali metals dissolve in liquid ammonia to form deep blue solution.