 Long Answer Type
Long Answer TypeLithium is the strongest reducing agent in aqueous solution. Explain.
Electrode potential is the measure of the tendency of an element to lose electrons in the aqueous solution. Thus, more negative is the electrode potential, higher is the tendency of the element to lose electrons and hence stronger is the reducing agent.
Since the standard electrode potential  of
of
alkali metals become more and more negative as we move down the group from Na to Cs, therefore, reducing character of these elements increases in the same order i.e. Na to Cs. However, standard electrode potential (reduction) of lithium is the lowest i.e. -3.05 volts. In other words, lithium is the strongest reducing agent in the aqueous solution.
Reason. Electrode potential depends on :
(i) heat of sublimation
(ii) ionisation enthalpy
(iii) heat of hydration.
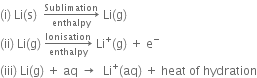
The sublimation enthalpies of alkali metals are almost similar. Now since Li+ ion is smallest in size, therefore, the large amount of energy released in step III (heat of hydration) compensates for the higher ionisation enthalpies, thereby facilitating the release of electron and hence explains the low value of electrode potential 
 Short Answer Type
Short Answer TypeWhy ionic conductance of alkali metal ions in aqueous solution are in the order:
Li+ < Mg + < K+ < Rb+ < Cs+ Explain ?
Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
Comment on each of the following observations:
The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ < K+ < Rb+ < Cs+
Comment on each of the following observations:
Lithium is the only alkali metal which forms nitride directly.
 Long Answer Type
Long Answer TypeLithium forms normal oxide, sodium forms peroxides while K, Rb and Cs form superoxides. Explain.
 Short Answer Type
Short Answer Type