 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhy ionic conductance of alkali metal ions in aqueous solution are in the order:
Li+ < Mg + < K+ < Rb+ < Cs+ Explain ?
Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
Comment on each of the following observations:
The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ < K+ < Rb+ < Cs+
Comment on each of the following observations:
Lithium is the only alkali metal which forms nitride directly.
 Long Answer Type
Long Answer TypeLithium forms normal oxide, sodium forms peroxides while K, Rb and Cs form superoxides. Explain.
Lithium forms normal oxide  Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Sodium reacts with dioxygen to form sodium peroxide 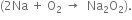 Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion
Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion 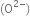 into a peroxide ion
into a peroxide ion 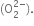 , However, it is strong enough to prevent further oxidation of peroxide to superoxide.
, However, it is strong enough to prevent further oxidation of peroxide to superoxide.
Potassium, rubidium and caesium react with dioxygen to form superoxide 
Potassium, rubidium and caesium ions are large sized and thus have a very weak positive field around them. The positive field around these ions is so weak that it cannot prevent the conversion of peroxide 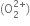 anion to superoxide anion
anion to superoxide anion 
 Short Answer Type
Short Answer Type