 Short Answer Type
Short Answer TypeComplete the following equations:
(i) 2Li + NH3 
(ii) 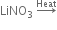
(iii) 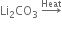
(iv) 
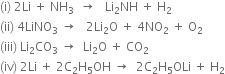
Write two properties of lithium carbonate in which it differs from other alkali metal carbonates.
Why is LiF almost insoluble in water whereas LiCl in soluble not only in water but also in acetone?
How would you explain that the following observations?
LiI is more soluble than KI in ethanol?
 Long Answer Type
Long Answer TypeExplain: Lithium exhibits anamalous behaviour in the company of alkali metals.
Or
Name the chief factor responsible for the anomalous behaviour of lithium.
Or
List three properties of lithium in which it differs from the rest of the alkali metals.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeIn what ways lithium shows similarities to magnesium in its chemical behaviour?
Or
List four properties to show the diagonal relationship between lithium and magnesium.
 Short Answer Type
Short Answer Type