 Long Answer Type
Long Answer TypeDiscuss the extraction of lithium.
It involves the following steps:
(i) Preparation of lithium chloride: The silicate mineral is crushed to fine powder and then boiled with dilute H2SO4 and filtered to remove insoluble silica (SiO2). The mother liquor is then treated with calculated amount of Na2CO3 to precipitate aluminium and iron as carbonates which are filtered off. The filtrate is then treated with an excess of Na2CO3 to precipitate lithium as Li2CO3. The precipitate is dissolved in HCl to form LiCl.
(ii) Electrolysis of lithium chloride. A mixture of dry lithium chloride and potassium chloride is fused and electrolyzed in an electrolytic cell (Down’s cell). Potassium chloride is added to increase the electrical conductivity and also to lower the melting point from 883K to 723K. The cell is operated at a temperature of about 675-775K and voltage of 8-9 volts is applied.
As a result of electrolysis, the following reactions take place: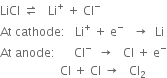
Chlorine gas is liberated at the anode while molten lithium rises to the surface of the fused electrolyte and collects in the cast iron enclosure surrounding the cathode. The metal thus obtained is 99% pure.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat happens when:
(i) Lithium reacts with air
(ii) Lithium reacts with water
(iii) Lithium reacts with halogen
(iv) Lithium reacts with acids?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Lithium can not form monovalent cation (Li+) easily.
(ii) Lithium iodide is more covalent than lithium fluoride.
 Long Answer Type
Long Answer TypeWhat difficulities arise in the extraction of sodium? How these difficulties are overcome?
 Short Answer Type
Short Answer TypeWhat happens when:
(i) sodium reacts with hydrogen halide,
(ii) sodium reacts with acetylene,
(iii) sodium is heated with hydrogen and
(iv) sodium is treated with mercury ?
