 Long Answer Type
Long Answer TypeWhat happens when:
(i) sodium metal is dropped in water?
(ii) sodium metal is heated in free supply of air?
(iii) sodium peroxide dissolves in water?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Sodium imparts colour to the flame.
(ii) Sodium acts as a strong reducing agent.
 Long Answer Type
Long Answer TypeDiscuss the various reactions which occur in the Solvay ammonia process.
In this process, brine (i.e. a concentrated solution of NaCl), ammonia and carbondioxide are the raw materials. The chemical reactions involved are: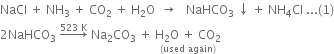
CO2 needed for the reaction is obtained by heating calcium carbonate and quick lime (CaO) is dissolved in water to form slaked lime Ca(OH)2.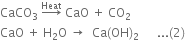
NH3 needed for the reaction is obtained by heating NH4Cl formed in eq. (1) with Ca(OH)2 formed in eq. (2).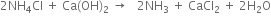
The only by product of the reaction is calcium chloride (CaCl2).
 Short Answer Type
Short Answer Type