 Short Answer Type
Short Answer TypeWhat happens when:
(i) sodium carbonate reacts with the milk of lime.
(ii) sodium carbonate is added to water.
(iii) sodium carbonate reacts with a dilute mineral acid?
 Long Answer Type
Long Answer TypeHow is sodium hydroxide manufactured? Discuss in brief the details of the process.
Or
With the help of a diagram, show the reactions at the cathode and anode in the manufacture of sodium hydroxide by the Castner - Kellner process.
 Short Answer Type
Short Answer TypeExplain what happens when:
(i) Sodium hydrogen carbonate is heated
(ii) Sodium amalgam reacts with water
(iii) Fused sodium metal reacts with ammonia?
(i) When sodium hydrogen carbonate is heated, sodium carbonate is formed.

(ii) Sodium amalgam reacts with water liberating hydrogen gas.
(iii) When ammonium is passed through molten sodium, it yields sodamide evolving H2 gas.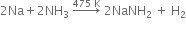
Explain the significance of sodium, potassium, calcium and magnesium in biological fluids ?
