 Short Answer Type
Short Answer TypeBeryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Comment on each of the following observations: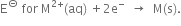
(where M = Ca, Sr or Ba) is nearly constant.
Explain why alkaline earth metals are poor reducing agents as compared to alkali metals.
The ionisation enthalpies of alkaline earth metals are higher and their electrode potentials are less negative than the corresponding alkali metals, therefore alkaline earth metals are weaker reducing agents than alkali metals.
 Long Answer Type
Long Answer TypeExplain the trend of solubility of carbonate, sulphates and hydroxides of alkaline earth metals ?
 Short Answer Type
Short Answer TypeAccount for the following:
(i) Be(OH)2 is amphoteric while Mg(OH)2 is basic.
(ii) Be(OH)2 is insoluble but Ba(OH)2 is fairly soluble in water.
