 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat is meant by the diagonal relationship of elements? Discuss the diagonal relationship of beryllium with aluminium.
Or
Beryllium exhibits some similarities with aluminium. Point out three such properties.
Diagonal relationship of beryllium with aluminium:
Since beryllium (Be2+) and aluminium (Al3+) have similar charge/radius ratio, they exhibit diagonal relationship. They resemble as follows:
(i) Both these elements dissolve in strong alkalies to liberate hydrogen and forming beryllate's and aluminates.
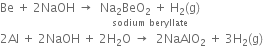
(ii) Both have strong tendency to form covalent compounds. Both BeCl2 and AlCl3 are covalent.
(iii) Both form non-volatile, hard oxides (BeO and Al2O3) having very high melting points.
(iv) Both Be and Al form fluoro-complex anions 
(v) Carbides of both the metals react with water liberating methane gas.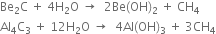
Discuss the diagonal realtionship of Be and Al with regard to:
(i) action of alkali (ii) structure of chlorides.
 Short Answer Type
Short Answer TypeGive any three points of similarities between beryllium and aluminium and two points of dissimilarities between beryllium and barium ?
 Long Answer Type
Long Answer TypeHow does magnesium occur in nature? How is magnesium obtained by electrolysis method ?
What happens when:
(i) magnesium is heated with water.
(ii) magnesium is heated in an atmosphere of carbon dioxide,
(iii) magnesium is treated with dilute sulphuric acid and
(iv) magnesium is treated with nitrogen?
