 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat is meant by the diagonal relationship of elements? Discuss the diagonal relationship of beryllium with aluminium.
Or
Beryllium exhibits some similarities with aluminium. Point out three such properties.
Discuss the diagonal realtionship of Be and Al with regard to:
(i) action of alkali (ii) structure of chlorides.
 Short Answer Type
Short Answer TypeGive any three points of similarities between beryllium and aluminium and two points of dissimilarities between beryllium and barium ?
 Long Answer Type
Long Answer TypeDraw the structure of: (i) BeCl2 (vapour) (ii) BeCl2 (solid).
In the solid state, BeCl2 has polymeric chain structure. Be atom is tetrahedrally surrounded by four Cl atoms - two are bonded by covalent bonds while the other two by coordinate bonds. The polymeric structure of BeCl2 is due to its electron deficient nature. It has only four electrons in valence shell and can accept two pairs of electrons from neighbouring chlorine atoms to complete their octet.![]()
In the vapour state, beryllium chloride exists as a dimer (Be2Cl4) which dissociates at 1200 K into monomer (BeCl2) which has a linear shape.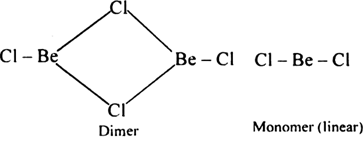
How does magnesium occur in nature? How is magnesium obtained by electrolysis method ?
What happens when:
(i) magnesium is heated with water.
(ii) magnesium is heated in an atmosphere of carbon dioxide,
(iii) magnesium is treated with dilute sulphuric acid and
(iv) magnesium is treated with nitrogen?
