 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat is meant by the diagonal relationship of elements? Discuss the diagonal relationship of beryllium with aluminium.
Or
Beryllium exhibits some similarities with aluminium. Point out three such properties.
Discuss the diagonal realtionship of Be and Al with regard to:
(i) action of alkali (ii) structure of chlorides.
 Short Answer Type
Short Answer TypeGive any three points of similarities between beryllium and aluminium and two points of dissimilarities between beryllium and barium ?
 Long Answer Type
Long Answer TypeHow does magnesium occur in nature? How is magnesium obtained by electrolysis method ?
Magnesium docs do not occur in the free state in nature. In the combined state (minerals) it occurs as,
(i) Magnesite MgCO3
(ii) Dolomite MgCO3.CaCO3
(iii) Carnallite KCl MgCl2.6H2O
(iv) Epsom salt MgSO4.7H2O
All plants and animal tissues contain small amounts of magnesium, it is contained in chlorophyll, the green colouring matter of plants. Sea water contains an appreciable amount of magnesium chloride.
Extraction of magnesium: Magnesium is usually extracted by the electrolysis of fused magnesium chloride or carnallite. It is isolated form sea water by Dow process. The various steps employed are
(i) Precipitation of magnesium hydroxide: Sea water is treated with lime water when magnesium hydroxide gets precipitated.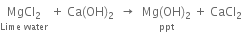
(ii) Conversion of magnesium hydroxide into magnesium chloride: The precipitate of magnesium hydroxide is dissolved in hydrochloric acid to get a clear solution of magnesium chloride.
The solution is concentrated when MgCl2.6H2O crystallises out.
(iii) Preparation of anhydrous magnesium chloride: A current of HCl gas is passed through magnesium chloride hexahydrate when anhydrous magnesium chloride is obtained.
(iv) Electrolysis of anhydrous magnesium chloride: Anhydrous magnesium chloride is added to a molten mixture of sodium chloride and calcium chloride (973 -1023 K). The mixture is then electrolyzed in an electrolytic cell which consists of the iron vessel which acts as a cathode. The anode consists of a graphite rod enclosed in a porcelain hood. The cell is heated externally to about 973 -1073 K. A stream of an inert gas such as coal gas is passed through the cell to check the oxidation of liberated magnesium by atmospheric oxygen. On passing electric current, the following reaction takes place:
At cathode. 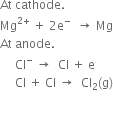
Thus, magnesium is liberated at the cathode while chlorine gas is liberated at the anode.
What happens when:
(i) magnesium is heated with water.
(ii) magnesium is heated in an atmosphere of carbon dioxide,
(iii) magnesium is treated with dilute sulphuric acid and
(iv) magnesium is treated with nitrogen?
