 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeHow is quick lime prepared on a commercial scale? How is it converted into slaked lime?
How will you distinguish between:
(i) Slaked lime (ii) Milk of lime (iii) Lime water?
(i) Slaked lime: It is a white amorphous solid formed when quicklime is added to water.
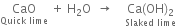
(ii) Milk of lime: It is the suspension of slaked lime in water.
(iii) Lime water: When milk of lime is kept for some time undisturbed in a beaker and the solution formed on the surface is decanted. It is called lime water.
 Long Answer Type
Long Answer TypeWhat is the effect of heat on the following compounds? (Write equations for the reactions):
(i) Calcium Carbonate
(ii) Magnesium chloride hexahydrate
(iii) Gypsum
(iv) Magnesium sulphate heptahydrate
 Short Answer Type
Short Answer Type