 Short Answer Type
Short Answer TypeEstimate the enthalpy change for the reaction:![]()
Given that bond enthalpy of H – H, Br – Br and H – Br bonds are 435 kJ mol–1, 192 kJ mol–1 and 368 kJ mol–1 respectively.
Calculate ![]() of tge reaction
of tge reaction
The average bond enthalpies of C – H bond and C – Cl bond are 415 kJ mol–1 and 326 kJ mol–1 respectively.
Compute the enthalpy of hydrogenation of ethylene if the bond enthalpies of H – H, C – H, C – C and C = C bonds are 436, 485, 347 and 619 kJ mol–1 respectively.
 Long Answer Type
Long Answer TypeCalculate ![]() for the reaction
for the reaction ![]()
The bond enthalpies of C - H, O = O, C = O, O - H and C = C bonds are 414, 499, 460 and 619 kJ mol-1 respectively.

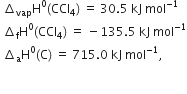
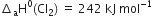
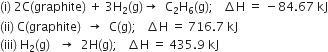
The enthalpies of combustion of methane and ethane are –890.3 and –1559.7 kJ mol–1 respectively. Which of the two has a greater efficiency of the fuel per gram?
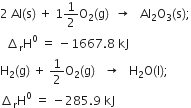
 Short Answer Type
Short Answer Type