Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeCalculate the maximum work obtained when 0.75 mol of an ideal gas expands isothermally and reversibly at 27°C from a volume of 15L to 25L.
If water vapour is assumed to be a perfect gas,
molar enthalpy change for vapourisation of 1 mol of water at 1bar and 100°C is 41kJ mol–1. Calculate the internal energy change, when
(i) 1 mol of water is vaporised at 1 bar pressure and 100°C.
 Long Answer Type
Long Answer TypeDerive mathematical form of First law of Thermodynamics.
Or
Derive the relationship between heat, internal energy and work.
Mathematical form:
The internal energy of the system can be changed in two ways:
(i) by allowing heat to flow into the system or out of the system,
(ii) by work is done on the system or work done by the system.
Let us consider a system which undergoes a change from one state to another. Let the initial internal energy of the system be U1. Now if a system is supplied q amount of heat, then internal energy of the system increases and becomes U1 + q.
If work (w) is done on the system, then its internal energy further increases and becomes U2. The energy U2 is the energy in the final state.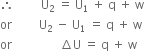
The above expression is the mathematical form of the first law of thermodynamics. w includes all kinds of work such as pressure-volume work, electrical work etc.
If the work is done in the above process is only pressure-volume work,