 Short Answer Type
Short Answer TypeThe enthalpy change (∆H) for the reaction
![]()
is -92.38 kJ at 298 K. what is the ![]() at 298 K?
at 298 K?
∆U⊝ of combustion of methane is –X kJ mol–1. The value of ∆H⊝ is :
(i) = ∆U⊝
(ii) > ∆U⊝
(iii) < ∆U⊝
(iv) = 0
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWhat do you understand by:
(i) Heat capacity of a substance
(ii) Heat capacity at constant volume
(iii) Heat capacity at constant pressure?
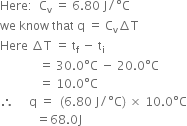
60.8 J of energy is required to change the temperature of 25.0 g of ethylene glycol (a compound used as an antifreeze in automobile engines) by 1.0 K. Calculate heat capacity of ethylene glycol.
