 Short Answer Type
Short Answer TypeCalculate the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at –10.0°C.
Heat capacity at constant pressure is greater than heat capacity at constant volume. Why?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type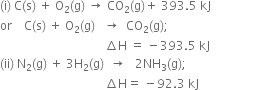
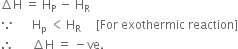
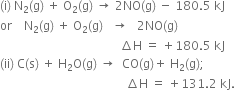


Comment on:
(i) ∆U is –ve for exothermic reactions and
(ii) ∆U is +ve for endothermic reactions.
 Short Answer Type
Short Answer TypeGive the points of difference between exothermic reactions and enodthermic reactions.
 Long Answer Type
Long Answer Type