 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type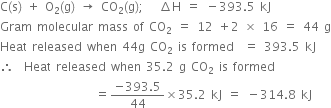
How much heat is evolved when 204g of ammonia are produced according to the equation,![]()
 Long Answer Type
Long Answer TypeExplain:
(i) Enthalpy (heat) of the formation.
(ii) Standard enthalpy of formation. How is it helpful in calculating the enthalpy of a reaction?
