 Short Answer Type
Short Answer TypeA swimmer coming out from a pool is covered with a film of water weighing about 80 g. How much heat must be supplied to evaporate this water?
When 1 gm of liquid naphthalene (C10H8) solidifies, 49 joules of heat is evolved. Calculate the enthalpy of fusion of naphthalene.
 Long Answer Type
Long Answer TypeHow many grams of methane and volume of oxygen at N.T.P. would be required to produce 445.15 kJ of heat in the following reaction?
                                                          
                                       
The thermochemical reaction is,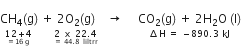
Step I. Amount of methane required:
890.3 kJ of heat is produced from methane = 16 g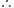  445.15 kJ of heat is produced from methane
 445.15 kJ of heat is produced from methane
                                        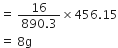
Step II. Volume of oxygen required:
890.3 kJ heat is evolved from oxygen = 44.8 litres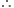  445.15 kJ of heat is evolved from oxygen
 445.15 kJ of heat is evolved from oxygen
                                   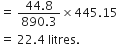
Standard vaporisation enthalpy of benzene at its boiling point is 30.8 kJ mol-1 ; for how long would a 100W electric heater has to operate in order to vaporise a 100g sample of benzene at its boiling temperature?
 Short Answer Type
Short Answer TypeThe thermochemical equations for the formation of water are:
                                            

How much energy is needed to convert 2 mol of liquid water to water vapour?
