 Long Answer Type
Long Answer TypeCalculate the heat change accompanying the transformation of C(graphite) to C(diamond). You are given:
The equation,
Given that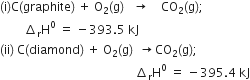
Subtracting equation (ii) from equation (i), we have, 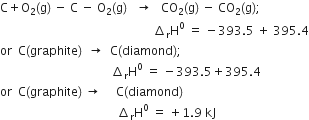
i.e. 1.9 kJ of heat is absorbed in the process.

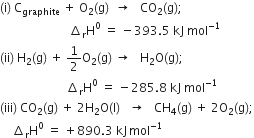
The combustion of one mole of benzne takes place at 298K and 1 atm. After combustion, CO2(g) and H2O (l) are produced and 3267.0 kJ of heat is liberated. Calculate the standard enthalpy of formation, ∆fH° of benzene. Standard enthalpies of formation of CO2(g) and H2O(l) are – 393.5 kJ mol–1 and –285.83 kJ mor–1respectively.
What do you mean by bond enthalpy? When is bond enthalpy equal to bond dissociation energy?
 Short Answer Type
Short Answer Type