 Long Answer Type
Long Answer TypeCalculate the heat change accompanying the transformation of C(graphite) to C(diamond). You are given:

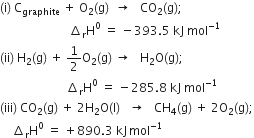
We aim at the equation
Given that: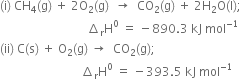
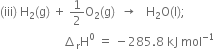
Multiplying equation (iii) by 2, we have
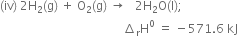
Adding equations (ii) and (iv),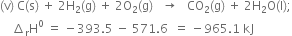
Subtracting equation (i) from equation (v), we have
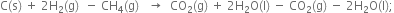
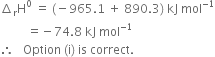
The combustion of one mole of benzne takes place at 298K and 1 atm. After combustion, CO2(g) and H2O (l) are produced and 3267.0 kJ of heat is liberated. Calculate the standard enthalpy of formation, ∆fH° of benzene. Standard enthalpies of formation of CO2(g) and H2O(l) are – 393.5 kJ mol–1 and –285.83 kJ mor–1respectively.
What do you mean by bond enthalpy? When is bond enthalpy equal to bond dissociation energy?
 Short Answer Type
Short Answer Type