 Short Answer Type
Short Answer TypeEstimate the enthalpy change for the reaction:![]()
Given that bond enthalpy of H – H, Br – Br and H – Br bonds are 435 kJ mol–1, 192 kJ mol–1 and 368 kJ mol–1 respectively.
Calculate ![]() of tge reaction
of tge reaction
The average bond enthalpies of C – H bond and C – Cl bond are 415 kJ mol–1 and 326 kJ mol–1 respectively.
Compute the enthalpy of hydrogenation of ethylene if the bond enthalpies of H – H, C – H, C – C and C = C bonds are 436, 485, 347 and 619 kJ mol–1 respectively.
 Long Answer Type
Long Answer TypeCalculate ![]() for the reaction
for the reaction ![]()
The bond enthalpies of C - H, O = O, C = O, O - H and C = C bonds are 414, 499, 460 and 619 kJ mol-1 respectively.

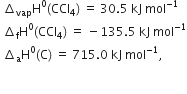
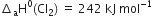
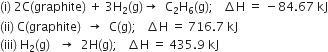
What do you mean by calorific value of foods and fuels?
Coal, petroleum, natural gas etc. are regarded as fuels for many machines and industrial purposes. The oxidation or combustion of all these fuels releases energy. This energy is measured in terms of calorific value. It is defined as the amount of heat produced by the complete combustion of one gram of the substance (food or fuel) in oxygen. It is generally expressed in kilocalories per gram (k cal g–1) or kJ g–1. The calorific value of fuel or food is measured by bomb calorimeter. Greater the calorific value, better is the fuel.
Food is regarded as fuel for the animal or human body. It has been found that fats and carbohydrates are the main sources of energy which have calorific values of about 9 k cal g–1 and 4 k cal g–1 respectively. Proteins are also the main constituents of our food but have a low calorific value of 4 k cal g–1.
The enthalpies of combustion of methane and ethane are –890.3 and –1559.7 kJ mol–1 respectively. Which of the two has a greater efficiency of the fuel per gram?
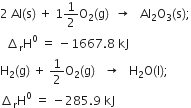
 Short Answer Type
Short Answer Type