 Short Answer Type
Short Answer TypeEstimate the enthalpy change for the reaction:![]()
Given that bond enthalpy of H – H, Br – Br and H – Br bonds are 435 kJ mol–1, 192 kJ mol–1 and 368 kJ mol–1 respectively.
Calculate ![]() of tge reaction
of tge reaction
The average bond enthalpies of C – H bond and C – Cl bond are 415 kJ mol–1 and 326 kJ mol–1 respectively.
Compute the enthalpy of hydrogenation of ethylene if the bond enthalpies of H – H, C – H, C – C and C = C bonds are 436, 485, 347 and 619 kJ mol–1 respectively.
 Long Answer Type
Long Answer TypeCalculate ![]() for the reaction
for the reaction ![]()
The bond enthalpies of C - H, O = O, C = O, O - H and C = C bonds are 414, 499, 460 and 619 kJ mol-1 respectively.

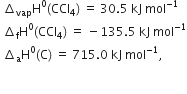
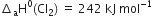
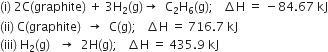
The enthalpies of combustion of methane and ethane are –890.3 and –1559.7 kJ mol–1 respectively. Which of the two has a greater efficiency of the fuel per gram?
The fuel efficiency can be predicted from the amount of heat evolved for every gram of fuel consumed.
(i) Thermochemical equation for the combustion of methane is,
Gram molecular mass of methane 
= 12 + 4 X 1 = 16 g
(ii) Thermochemical equation for combustion of ethane is
Gram molecular mass of ethane 
= 2 x 12 + 6 x 1 = 30 g
Thus, methane has greater fuel efficiency than ethane.
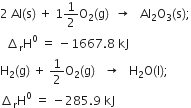
 Short Answer Type
Short Answer Type