 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeIn 1919, Born and Haber proposed a method in which lattice enthalpy of an ionic crystal is related to certain thermodynamic parameters. The formation of ionic crystal from its elements by applying different thermochemical quantities is called Born-Haber cycle.
Consider the enthalpy change during the formation of ionic solid MX from its elements M and X.
Formation of ionic solid MX may be done by two different methods:
(A) Indirect method
(B) Direct method
(A) Indirect method:
The various terms are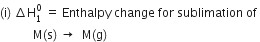
Since energy is required for the process (endothermic), therefore,  is taken as a positive quantity.
is taken as a positive quantity.
Since the process is endothermic, therefore ionisation enthalpy is taken as a positive quantity.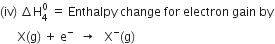
Since the process is exothermic, therefore electron gain enthalpy is taken as negative quantity.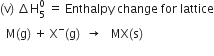
Energy is released in this process i.e. process is exothermic, therefore ![]() is always taken as negative quantity.
is always taken as negative quantity.
Direct method: Let ![]() be the enthalpy of formation of 1 mol of MX(s) from its constituent species.
be the enthalpy of formation of 1 mol of MX(s) from its constituent species.
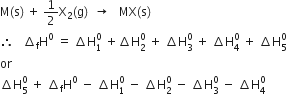
where ![]() is the enthalpy change for lattice formation from,
is the enthalpy change for lattice formation from,

The reverse of the above equation is

Thus by using the Born-Haber cycle, one can determine the lattice enthalpy of an ionic compound.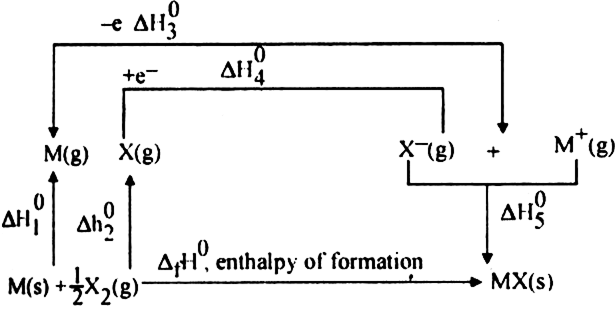
According to Hess’s law, the enthalpy of formation of one mole of ionic solid MX should be the same irrespective of the fact whether it takes place directly in one step (Direct method) or through a number of steps (Indirect method).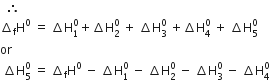
How can the lattice enthalpy of an ionic NaCl be determined by using Born-Haber cycle?
Calculate the lattice enthalpy of KCl crystal from the following data:
Sublimation enthalpy of pottasium (K) = +89 kJ mol-1
Dissociation enthalpy of ![]()
= +122 kJ mol-1
Ionisation enthalpy of K(g) +425 kJ mol-1
Electron gain enthalpy of Cl(g) = -355 kJ mol-1
Enthalpy of formation of ![]()
Calculate the lattice enthalpy of LiF, given that the enthalpy of
(i) sublimation of lithium is 155.2 kJ mol–1
(ii) dissociation of 1/2 mol of F2is 75.3 kJ
(iii) ionization of lithium is 520 kJ mol–1
(iv) electron gain of 1 mol of F(g) is –333 kJ
(v) ∆f H0 ∆fH0 overall is –795 kJ mol–1
 Short Answer Type
Short Answer TypeCalculate the lattice enthalpy of MgBr2 from the given data. The enthalpy of formation of MgBr2 according to the reaction

 Long Answer Type
Long Answer TypeThe tendency of a system to acquire a state of maximum randomness is the sole criterion for the spontaneity of a process. Comment.
