 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeHow can the lattice enthalpy of an ionic NaCl be determined by using Born-Haber cycle?
Formation of NaCl may be done by two different methods:
(a) Indirect method
(b) Direct method.
(a) Indirect method: Various terms involved are:
Since enthalpy is required for the process (endothermic), therefore  is taken as a positive quantity.
is taken as a positive quantity.
(ii) 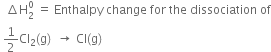
The process is endothermic, therefore ![]() is taken as a positive quantity.
is taken as a positive quantity. 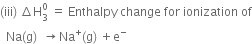
Since the process is endothermic, therefore, ionisation enthalpy is taken as a positive quantity.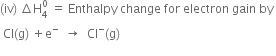
Since the process is exothermic, therefore electron gain enthalpy is taken as a negative quantity. ![]()
Enthalpy is released in this process i.e. process is exothermic, therefore ![]() is always taken as a negative quantity.
is always taken as a negative quantity.
(b) Direct method: Let ![]() be the enthalpy of formation of 1 mol of NaCl(s) from its constituent species.
be the enthalpy of formation of 1 mol of NaCl(s) from its constituent species.

According to Hess's law, the enthalpy of formation of one mole of sodium chloride should be the same irrespective of the fact whether it takes place directly in one step.
(Direct method) or through a number of steps (Indirect method).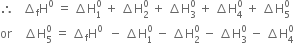
where  is the enthalpy change for lattice formation from
is the enthalpy change for lattice formation from

The reverse of the above equation i.e.

defines the lattice enthalpy of NaCl.
Thus by using the Born- Haber cycle, one can determine the lattice enthalpy of an ionic compound.
Calculate the lattice enthalpy of KCl crystal from the following data:
Sublimation enthalpy of pottasium (K) = +89 kJ mol-1
Dissociation enthalpy of ![]()
= +122 kJ mol-1
Ionisation enthalpy of K(g) +425 kJ mol-1
Electron gain enthalpy of Cl(g) = -355 kJ mol-1
Enthalpy of formation of ![]()
Calculate the lattice enthalpy of LiF, given that the enthalpy of
(i) sublimation of lithium is 155.2 kJ mol–1
(ii) dissociation of 1/2 mol of F2is 75.3 kJ
(iii) ionization of lithium is 520 kJ mol–1
(iv) electron gain of 1 mol of F(g) is –333 kJ
(v) ∆f H0 ∆fH0 overall is –795 kJ mol–1
 Short Answer Type
Short Answer TypeCalculate the lattice enthalpy of MgBr2 from the given data. The enthalpy of formation of MgBr2 according to the reaction

 Long Answer Type
Long Answer TypeThe tendency of a system to acquire a state of maximum randomness is the sole criterion for the spontaneity of a process. Comment.
