 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeProve that ∆G = –T∆Stotal.
The change in entropy in a process carried out in a non-isolated system is given by
∆Stotal = ∆Ssystem + ∆Ssurroundings ...(1)
Consider an isothermal process carried out at constant pressure in which heat q is transferred by the surroundings to the system.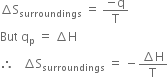
Substituting this value in (1), we have,

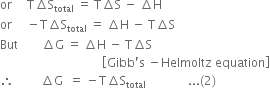
It has been shown that ![]() must be positive for a process to be spontaneous. Therefore, equation (2) becomes useful in predicting the spontaneity of a process in terms of ∆G.
must be positive for a process to be spontaneous. Therefore, equation (2) becomes useful in predicting the spontaneity of a process in terms of ∆G.
What is free energy change? Show that the change in free energy is equal to useful work done.
or
prove that –∆G = w(useful work)
 Short Answer Type
Short Answer TypePredict the enthalpy change, free energy change and entropy change when ammonium chloride is dissolved in water and the solution becomes colder.
At ![]() , ice and water are in equilibrium and
, ice and water are in equilibrium and ![]() for the process
for the process ![]() Calculate
Calculate ![]() for the conversion of ice to liquid water.
for the conversion of ice to liquid water.
For the reaction![]()
calculate ![]() at 700K when enthalpy and entropy changes are -113 kJ mol-1 and -145 JK-1 mol-1 respectively.
at 700K when enthalpy and entropy changes are -113 kJ mol-1 and -145 JK-1 mol-1 respectively.
 Long Answer Type
Long Answer TypeFrom the following values of ∆H and ∆S, decide whether or not these reactions will be spontaneous at 298 K:
Reaction A:
∆H = – 10.5 X 103 J mol–1
∆S = + 31 JK–1 mol–1
Reaction B:
∆H = – 11.7 X 103 J mol–1 ;
∆S = –105 jK–1 mol–1.
