 Short Answer Type
Short Answer TypeCalculate the free energy change of the reaction ![]()
![]()
According to Gibb's Helmoltz equation,

Here 

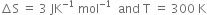
Substituting the values in (1), we have,
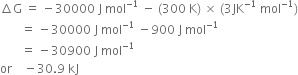
 Long Answer Type
Long Answer TypeAt what temperature, reduction of lead oxide to lead by carbon

becomes spontaneous? For this reason  are 108.4 kJ mol-1 and 190.0 JK-1 mol-1 respectively.
are 108.4 kJ mol-1 and 190.0 JK-1 mol-1 respectively. 
For the reaction at 298 K
![]()
![]()
At what temperature will the reaction become spontaneous considering ![]() to be constant over the temperature range?
to be constant over the temperature range?
 Short Answer Type
Short Answer TypeFor the reaction
![]()
Calculate ![]() for the reaction and predict whether the reaction may occur spontaneously.
for the reaction and predict whether the reaction may occur spontaneously.
 Long Answer Type
Long Answer TypeFor the reaction![<pre>uncaught exception: <b>Http Error #404</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php line 61<br />#0 [internal function]: com_wiris_plugin_impl_HttpImpl_0(Object(com_wiris_plugin_impl_HttpImpl), NULL, 'http://www.wiri...', 'Http Error #404')
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('com_wiris_plugi...', Array)
#2 [internal function]: _hx_lambda->execute('Http Error #404')
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(532): call_user_func_array(Array, Array)
#4 [internal function]: haxe_Http_5(true, Object(com_wiris_plugin_impl_HttpImpl), Object(com_wiris_plugin_impl_HttpImpl), Array, Object(haxe_io_BytesOutput), true, 'Http Error #404')
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('haxe_Http_5', Array)
#6 [internal function]: _hx_lambda->execute('Http Error #404')
#7 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(27): call_user_func_array(Array, Array)
#8 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(444): com_wiris_plugin_impl_HttpImpl->onError('Http Error #404')
#9 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(458): haxe_Http->customRequest(true, Object(haxe_io_BytesOutput), NULL, NULL)
#10 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(40): haxe_Http->request(true)
#11 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(80): com_wiris_plugin_impl_HttpImpl->request(true)
#12 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#13 {main}</pre>](/application/zrc/images/qvar/CHEN11088780.png)
![]() , calculate the temperature at which
, calculate the temperature at which ![]() is equal to zero. Also predict the direction of the reaction at:
is equal to zero. Also predict the direction of the reaction at:
(i) temperature
(ii) below this temperature
 Short Answer Type
Short Answer TypeFor the melting of ice at ![]()
![]()
the enthalpy of fusion is ![]() and entropy of fusion is 25.4
and entropy of fusion is 25.4 ![]() Calculate the free energy change and predict whether the melting of ice is spontaneous or not at this temperature.
Calculate the free energy change and predict whether the melting of ice is spontaneous or not at this temperature.
 Long Answer Type
Long Answer TypeWhat do you understand by:
(i) Standard free energy change (∆rG0)
(ii) Standard free energy of formation (∆fG0).
 Short Answer Type
Short Answer TypeCalculate the standard free energy change for the reaction![]()
Given that the standard free energies of formation ![]() for
for ![]() are -16.8, +86.7 and -237.2 kJ mol-1 respectively. Predict the feasibility of the above reaction at the standard state.
are -16.8, +86.7 and -237.2 kJ mol-1 respectively. Predict the feasibility of the above reaction at the standard state.
