 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeExplain the effect of temperature on feasibility for:
(i) endothermic process
(ii) exothermic process in terms of Gibb’s Helmoltz equation.
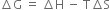
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type(a) What are Exergonic and Endergonic reactions?
(b) Given that the standard heat of formation of NH3(g) as represented by the equation![]()
is – 46.191 kJ. The standard entropies of N2(g), H2(g) and NH3(g) are 191.62, 130.12 and 193.3 JK–1 mol–1 respectively. Calculate the standard free energy of formation (∆G0) for NH3. Is the reaction feasible?
(i) Define first and the second law of thermodynamics in the combined form.
(ii)
(a) Write an expression for the entropy change of an ideal gas for isothermal change.
(b) Write an expression for entropy change for an ideal gas for isobaric change.
(iii) Calculate the maximum work obtained when 0.75 ml of an ideal gas expands isothermally and reversibly at 27°C from a volume of 15L to 25L.
(i) What are the limitations of criterion for randomness?
(ii) Calculate the standard free energy of formation of ![]() The free change for the reaction.
The free change for the reaction.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type