 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compounds will give red colour on Lassaigne test?
NaCNS
NH2CSNH2 (thiourea)
p-NH2C6H4SO3H (p- aminobenzene sulphonic acid)
all of the above
A positive carbylamine test is given by
I. N, N-dimethylaniline
II. 2, 4-dimethylamine
III. N-methyl-o-methylaniline
IV. p-methylbenzyl amine
(II) and (IV)
(I) and (IV)
(II) and (III)
(I) and (II)
The IUPAC name of the following compound is
![]()
2-carbamoylhexanal
2-carbamoylhex-3-enal
2-methyl-6-oxohex-3-enamide
6-keto-2-methyl hexamide
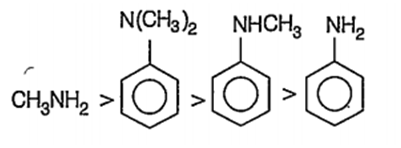
Which is the strongest base among the following?
H3CNH2
![]()
![]()
![]()
A.
H3CNH2
The species having more lone pair of electrons for donation are strong base.
In case of CH3NH2, one pair of electrons on N-atom is available for donation. Hence, itis most basic. Order of basic nature of the given compounds is
Cyclohexylamine and aniline can be distinguished by
Hinsberg test
Carbylamine test
Lassaigne test
Azo dye test
