 Multiple Choice Questions
Multiple Choice QuestionsMolecular formulae and shapes of some molecules are given below. Choose the incorrect match.
NH3 - Trigonal pyramidal
SF4 - Tetrahedral
ClF3 - T-shaped
PCl5 - Triginal bipyramidal
The calculated magnetic moment of a divalent ion of an atom with atomic number 24 in aqueus solution is
4.90 BM
5.92 BM
3.87 BM
2.84 BM
Molecular shapes of SF4 ,CF4 and XeF4 and the number of lone pairs on the central atom are respectively
the same, with 1, 2 and 1
the same with 1, 0 and 1
different with 0, 1 and 2
different with 1, 0 and 2
Which one of the following is not correct in respect of hybridization of orbitals?
The orbitals present in the valence shell are hybridized
The orbitals undergoing hybridization have akmost equal energy
Pure atomic orbitals are more effective in forming stable bonds than hybrid orbitals
It is not always that only partially filled orbitals participate in hybridization in some cases even filled orbitals in valence shell take part.
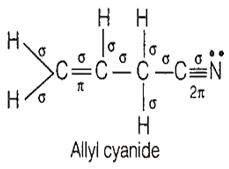
Allyl cyanide molecule contains
9 sigma bonds, 4 pi bonds and no lone pair
9 sigma bonds, 3 pi bonds and one lone pair
8 sigma bonds, 5 pi bonds and one lone pair
8 sigma bonds, 3 pi bonds and two lone pairs
B.
9 sigma bonds, 3 pi bonds and one lone pair
Allyl cyanide molecule consists of 9 sigma (σ), 3 pi () and 1 lone pair of electron.
Which one of the following statements is correct?
Ferromagnetic substance like ZnFe2O4 becomes paramagnetic on heating
NaCl is a paramagentic salt
CuSO4 is a diamagnetic salt
MnO is an example of antiferronmagnetic substance
In the dichromate dianion, the nature of bonds are
for equivalent Cr-O bonds
six equivalent Cr-O bonds and one O-O bond
six equivalent Cr-O bonds and one Cr-Cr bond
six equivalent Cr-O bonds and one Cr-O-Cr bond
Amongst the following ions which one has the highest magnetic moment value? (At. no. Co = 27, Ni = 28)
[Co(NH3)6]3+
[CoF6]3-
[NiCl4]2-
[Ni(CN)4]2-
Which among the following solid is a non-polar solid?
Hydrogen chloride
Sulphur dioxide
Water
Carbon dioxide
