 Multiple Choice Questions
Multiple Choice QuestionsGeometry of ammonia molecule and the hybridisation of nitrogen involved in it are
sp3-hybridisation and tetrahedral geometry
sp3-hybridisation and distorted tetrahedral geometry
sp2-hybridisation and triangular geometry
None of the above
Be in BeCl2 undergoes
diagonal hybridisation
trigonal hybridisation
tetrahedral hybridisation
no hybridisation
The catenation tendency of C, Si and Ge is in the order Ge < Si < C. The bond energies (in kJ mol-1) of C-C, Si-Si and Ge-Ge bonds, respectively are
167, 180, 348
180, 167, 348
348, 167, 180
348, 180, 167
The number of lone pairs of electrons present on : Xe in XeF4 is
3
4
1
2
D.
2
Xe in ground state → 5s2, 5p6, 5d0
Xe in IInd excited state → 5s2, 5p4, 5d2
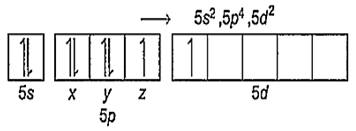
Number of lone pairs = 2
Number of bond pairs = 4
Therefore, the number of lone pairs of electrons present on Xe in XeF4 are 2.
Which of the following statements is true?
Hybridisation of the central atom in NH3 and CH4 is sp2
BeCl2 has V shape while SO2 is linear
SF6 is octahedral and F-S-F bond angle is 90°
CO2 has dipole moment
Bond dissociation energies of HF, HCl, HBr follow the order
HCl > HBr > HF
HF > HBr > HCl
HF > HCl > HBr
HBr > HCl > HF
Which one ofthe following is a correct set?
H2O, sp3, angular
H2O, sp2, linear
NH4+, dsp2,square planar
CH4, dsp2, tetrahedral
The bond energies (in kJ mol-1) of P-H; As- H and N-H are respectively
247, 389 and 318
247, 389 and 318
318, 389 and 247
318, 247 and 389
