 Multiple Choice Questions
Multiple Choice QuestionsFluorine reacts with dilute NaOH and forms a gaseous product A. The bond angle in the molecule of A is
104°40'
103°
107°
109°28'
The number of p-d 'pi' bonds present in XeO3 and XeO4 molecules, respectively are-
3, 4
4, 2
2, 3
3, 2
Which one of the following sets correctly represents the increase in the paramagnetic property of the ions?
Cu2+ > V2+ > Cr2+ > Mn2+
Cu2+ < Cr2+ < V2+ < Mn2+
Cu2+ < V2+ < Cr2+ < Mn2+
V2+ < Cu2+ < Cr2+ < Mn2+
Dipole moment of HCl = 1.03 D, HI= 0.38 D. Bond length of HCl = 1.3 Å and HI = 1.6 Å. The ratio of fraction of electric charge, δ, existing on each atom in HCl and HI is
12 : 1
2.7 : 1
3.3 : 1
1 : 3.3
Match the following
| Column I (molecules) | Column II ( number of lone pair on central atom) | ||
| A | NH3 | 1 | Three |
| B | H2O | 2 | Two |
| C | XeF2 | 3 | Zero |
| D | CH4 | 4 | Four |
| 5 | One |
A B C D
5 1 3 2
A B C D
3 1 2 5
A B C D
5 1 2 3
A B C D
1 5 3 4
Identify the order in which the spin only magnetic moment (in BM) increases for the following four ions
(I) Fe2+
(II) Ti2+
(III) Cu2+
(IV) V2+
I, II, IV, III
IV, I, II, III
III, IV, I, II
III, II, IV, I
The formal charges of N(1), N(2) and O atoms in the following figure are respectively
![]()
+1, -1, 0
-1, +1, 0
+1, +1, 0
-1, -1, 0
In which of the following pairs, the central atoms have the same number of lone pairs of electrons?
PCl5, BrF5
XeF2, ICl
XeF4, ClO
SCl4, CH4
B.
XeF2, ICl
Both XeF2 and ICl molecule have three lone pairs of electron on central atom.

Hence, in XeF2 three lone pairs are present.
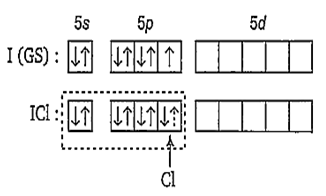
Hence, ICl three lone pairs are present.
Identify the correct set.
| Molecule | Hybridisation of central atom | Shape |
| PCl5 | dsp3 | square pyramidal |
| [Ni(CN)4]2- | sp3 | tetrahedral |
| SF6 | sp3d2 | octahedral |
| IF3 | dsp3 | pyramidal |
Which one of the following statements is correct?
Hybrid orbitals do not form σ bonds.
Lateral overlap of p-orbitals or p- and d-orbitals produces -bonds.
The strength of bonds follows the order-
σp-p < σs-s < p-p
s-orbitals do not form σ bonds.
