 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following is an electron deficient compound?
B2H6
NH3
C2H6
CCl4
A.
B2H6
B2H6 is an electron deficient compound in which B is sp3-hybridised state. It has the following H-bridged structure.
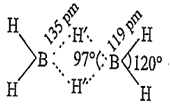
The shape of water molecule according to VSEPR theory, is
octahedral
distorted tetrahedral
trigonal planar
trigonal bipyramidal
In which of the following pairs both molecules do not possess same type of hybridisation?
CH4 and H2O
PCl5 and SF4
SF6 and XeF4
BCl3 and NCl3
If H-X bond length is 2.00 Å. and H-X bond has dipole moment 5.12 x 10-30 C-m, the percentage of ionic character in the molecule will be
100%
16%
18%
20%
(i) H—C—H angle in CH4
(ii) Cl—B—Cl angle in BCl3
(iii) F—I—F angle in IF7 in a plane
(iv) I—I—I angle in I
Increasing order of above bond angles is
(i) < (ii)< (iii)< (iv)
(ii)< (i) < (iii)< (iv)
(iii)< (i)< (ii)< (iv)
(iv)< (ii) < (i) < (iii)
