 Multiple Choice Questions
Multiple Choice QuestionsPick out the isoelectronic structures from the following
I and II
I and III
I and IV
II, III and IV
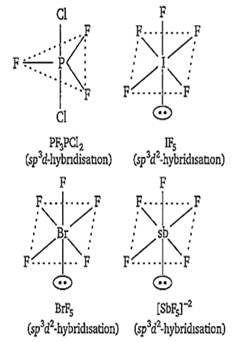
Which of the following will have the shape of a trigonal bipyramid?
PF3Cl2
IF5
BrF5
SbF
A.
PF3Cl2
PF3Cl2 has trigonal bipyramidal structure, IF5 BrF5 and SbF have octahedral geometries with one position occupied by a lone pair of electrons (square pyramidal geometry).
The bond present in N2O5 are
only ionic
covalent and coordinate
only covalent
covalent and ionic
Which of the following statements is false?
1. Non-bonding pairs occupy more space than bonding pairs.
2. The bonding orbitals in trigonal bipyramidal molecule are described as sp3d hybrids.
3. SnCl2 has linear shape.
4. PCl and AlCl are isoelectronic.
1
2
3
4
The correct order of increasing C-O bond length of CO, CO2 and CO is
CO < CO2 < CO
CO2 < CO < CO
CO < CO < CO2
CO < CO2 < CO
Covalent compounds have low melting point because
covalent molecules are held by weak van der Waal's force of attraction
covalent bond is less exothermic
covalent bond is weaker than ionic bond
covalent molecules have definite shape
