 Multiple Choice Questions
Multiple Choice QuestionsThe molecule C3O2 linear structure. This compound has
2σ and 3π bonds
3σ and 4π bonds
4σ and 4π bonds
3σ and 2π bonds
The structure of XeF2 and NH3 respectively are
linear, see-saw
bent, see-saw
bent, tetrahedral
linear, pyramidal
Which one of the following has (have) octahedral geometry?
(i) SbCl
(ii) SnCl
(iii) XeF6
(iv) IO
(i), (ii) and (iv)
(ii), (iii) and (iv)
(i), (ii) and (iii)
All of these
Among the following compounds of boron, the species which also forms -bond in addition to σ-bonds is
BH3
B2H6
BF3
BF
Number of bonding electron pairs and number of lone pairs of electrons in ClF3, SF4, BrF5 respectively are
3, 2, 4, 2, 5, 2
3, 1, 4, 1,5, 2
3, 1, 4, 2, 5, 1
3, 2, 4, 1, 5, 1
Match the following
| List I | List II |
| (a) PCl3 | (i) Square planar |
| (b) BF3 | (ii) T-shape |
| (c) ClF3 | (iii) Trigonal pyramidal |
| (d) XeF4 | (iv) See- saw |
| (v) Trigaonal planar |
(a) (b) (c) (d)
(iv) (ii) (i) (iii)
(a) (b) (c) (d)
(iii) (v) (ii) (iv)
(a) (b) (c) (d)
(iii) (v) (ii) (i)
(a) (b) (c) (d)
(iii) (v) (ii) (v)
The hybridisation of each carbon in the following compound respectively is CH3-CO-CH2CN
sp3,sp2,sp3,sp
sp3,sp3,sp2,sp
sp3,sp2,sp, sp3
sp2,sp, sp3, sp3
A.
sp3,sp2,sp3,sp
In the compound,
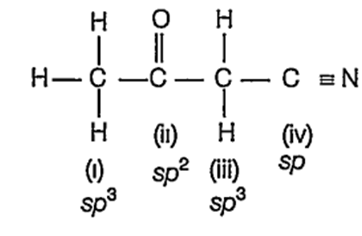
The C-atoms that is bonded to four different atoms involve four orbitals (1 of s and 3 of p- orbitals) during hybridisation. Hence, they have sp3 -hybridisation as in cases of (i) and (iii).
The C- atom bonded to three different atoms involves three orbitals (1 of s and 2 ofp-orbitals) Hence, the sp2-hybridisation in case of (ii).
The C-atom bonded to two different atoms involves two orbitals (1 of s and 1 of p-orbitals). Hence, the sp- hybridisation in case of (iv).
