 Multiple Choice Questions
Multiple Choice QuestionsFor the reaction, N2 + 3H2 → 2NH3, If 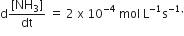 the value of
the value of 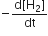 would be
would be
3 x 10-4 mol L-1 s-1
4 x 10-4 mol L-1s-1
6 x 10-4 mol L-1s-1
6 x 10-4 mol L-1s-1
In the reaction,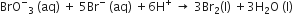
The rate of appearance of bromine (Br)2 is related to rate of disappearnace of bromide ions as following
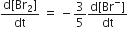

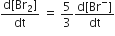
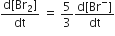
A.
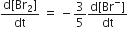
Rate of appearance/disappearance
For reaction,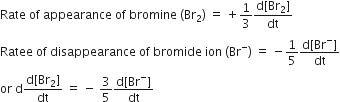
The rate constant k1 and k2 for two different reactions are 1016. e-2000/T and 1015.e-1000/T respectively. The temperature at which k1 = k2
1000 K
2000/2.303 K
2000 K
2000 K
The bromination of acetone cytosine and guanine solution is represented by this equation.
CH3COCH3 (aq) + Br2 (aq) →CH3COCH2Br (aq) + H+ (aq) + Br- (aq)
These kinetic data were obtained for given reaction concentrations.
|
Initial Concentrations, M
|
||
| [CH3COOH] | [Br2] | [H+] |
| 0.30 | 0.05 | 0.05 |
| 0.30 | 0.10 | 0.05 |
| 0.30 | 0.10 | 0.10 |
| 0.40 | 0.05 | 0.20 |
Rate = k[CH3COCH3][H+]
Rate = k[CH=COCH3][Br2]
Rate = k [CH3COCH3][Br2][H+]
Rate = k [CH3COCH3][Br2][H+]
The reaction of hydrogen and iodine monochloride is given as:
H2 (g) + 2ICl (g) → 2 HCl (g) + I2 (g)
This reaction is of first order with respect to H2 (g) and ICI (g), following mechanisms were proposed:
Mechanism A:
H2 (g) + 2 ICl (g) → 2 HCl (g) + I2 (g)
Mechanism B:
H2 (g) + ICl (g) →HCl (g) + HI (g) ;slow
HI (g) + ICl (g) → HCl (g) + I2 (g); fast
Which of the above mechanism (s) can be consistent with the given information about the reaction?
B only
A and B both
Neither A nor B
Neither A nor B
In a first order reaction A →B, if k israte constant and initial concentration of the reactant A is 0.5 A M then the half -life is :
0.693/0.5 k
log2/k
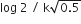
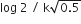
If 60% of a first order reaction was completed in 60 min, 50% of the same reaction would be completed in approximately:
50 min
45 min
60 min
60 min
For the reaction
2A + B → 3C + D
Which of the following does not express the reaction rate?




Consider the reaction
N2 (g) + 3 H2 (g) → 2 NH3 (g)
The equality relationship between  is:
is:




Which one of the following statements is not correct?
Catalyst does not initiate any reaction
The value of equilibrium constant is changed in the presence of a catalyst in the reaction at equilibrium
Enzymes catalyse mainly bio-chemical reactions
Enzymes catalyse mainly bio-chemical reactions
