 Multiple Choice Questions
Multiple Choice QuestionsFor the reaction, N2 + 3H2 → 2NH3, If 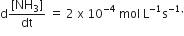 the value of
the value of 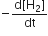 would be
would be
3 x 10-4 mol L-1 s-1
4 x 10-4 mol L-1s-1
6 x 10-4 mol L-1s-1
6 x 10-4 mol L-1s-1
In the reaction,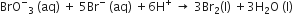
The rate of appearance of bromine (Br)2 is related to rate of disappearnace of bromide ions as following
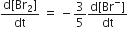

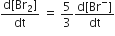
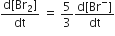
The rate constant k1 and k2 for two different reactions are 1016. e-2000/T and 1015.e-1000/T respectively. The temperature at which k1 = k2
1000 K
2000/2.303 K
2000 K
2000 K
The bromination of acetone cytosine and guanine solution is represented by this equation.
CH3COCH3 (aq) + Br2 (aq) →CH3COCH2Br (aq) + H+ (aq) + Br- (aq)
These kinetic data were obtained for given reaction concentrations.
|
Initial Concentrations, M
|
||
| [CH3COOH] | [Br2] | [H+] |
| 0.30 | 0.05 | 0.05 |
| 0.30 | 0.10 | 0.05 |
| 0.30 | 0.10 | 0.10 |
| 0.40 | 0.05 | 0.20 |
Rate = k[CH3COCH3][H+]
Rate = k[CH=COCH3][Br2]
Rate = k [CH3COCH3][Br2][H+]
Rate = k [CH3COCH3][Br2][H+]
The reaction of hydrogen and iodine monochloride is given as:
H2 (g) + 2ICl (g) → 2 HCl (g) + I2 (g)
This reaction is of first order with respect to H2 (g) and ICI (g), following mechanisms were proposed:
Mechanism A:
H2 (g) + 2 ICl (g) → 2 HCl (g) + I2 (g)
Mechanism B:
H2 (g) + ICl (g) →HCl (g) + HI (g) ;slow
HI (g) + ICl (g) → HCl (g) + I2 (g); fast
Which of the above mechanism (s) can be consistent with the given information about the reaction?
B only
A and B both
Neither A nor B
Neither A nor B
In a first order reaction A →B, if k israte constant and initial concentration of the reactant A is 0.5 A M then the half -life is :
0.693/0.5 k
log2/k
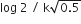
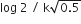
If 60% of a first order reaction was completed in 60 min, 50% of the same reaction would be completed in approximately:
50 min
45 min
60 min
60 min
For the reaction
2A + B → 3C + D
Which of the following does not express the reaction rate?




Consider the reaction
N2 (g) + 3 H2 (g) → 2 NH3 (g)
The equality relationship between  is:
is:




Which one of the following statements is not correct?
Catalyst does not initiate any reaction
The value of equilibrium constant is changed in the presence of a catalyst in the reaction at equilibrium
Enzymes catalyse mainly bio-chemical reactions
Enzymes catalyse mainly bio-chemical reactions
B.
The value of equilibrium constant is changed in the presence of a catalyst in the reaction at equilibrium
A catalyst decreases activation energies of both the forward and backward reaction by the same amount, therefore, it speeds up both forward and backward reaction by the same rate. The equilibrium constant is therefore not affected by the catalyst at a given temperature.
