 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of [Ni(PPh3)2Cl2]2+ is:
bis dichloro (triphenylphosphine) nickel (II)
dichloro bis (triphenylphosphine) nickel (II)
dichloro triphenylphosphine nickel (II)
triphenyl phosphine nickel (II) dichloride
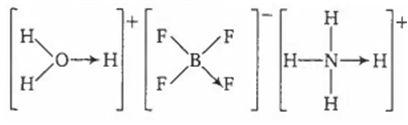
Which of the following does not contain any coordinate bond?
H3O+
C.
Rest all the molecules have coordinate bonds.
Select the diamagnetic complex ion amongst the following complexes (At. no. : Fe = 26,CO = 27)
K3[Fe(CN)6]
[Co(NH3)6)Cl3
K3[FeF6]
K3[CoF6]
The correct charge on and co-ordination number of 'Fe' in K3[Fe(CN)6] is
+2, 4
+3, 6
+2, 6
+3, 3
Which of the following co-ordinate complexes is an exception to EAN rule? (Given, atomic number Pt = 78, Fe = 26, Zn = 30, Cu = 29)
[Pt(NH3)6]4+
[Fe(CN)6]4-
[Zn(NH3)4]2+
[Cu(NH3)4]2+
Consider the following two complex ions : [CoF6]3- and [Co(C2O4)3]3-. Which of the following statement(s) is/are false?
I. Both are octahedral.
II. [Co(C2O4)3]3- is diamagnetic while [CoF6]3- is paramagnetic.
III. Both are outer orbital complexes.
IV. In both the complexes, the central metal is in the same oxidation state.
Both II and III
II, III and IV
Only III
Both III and IV
The hybridised state of Al3+ in the complex ion formed when AlCl3 is treated with aqueous acid is
sp3
dsp2
sp3d2
sp2d
Which of the following is a neutral complex?
[Pt(NH3)2Cl2]
[Co(NH3)6]Cl3
[Ni(NH3)6]Cl2
K4[Fe(CN)6]
