 Multiple Choice Questions
Multiple Choice QuestionsIn which one of the pairs of ion given, there is an ion that forms a coordination compound with both aqueous sodium hydroxide and ammonia and an other ion that forms a coordination compound only with aqueous sodium hydroxide?
Pb2+, Cu2+
Zn2+, Al3+
Cu2+, Zn2+
Al3+, Cu2+
The IUPAC name of the complex ion formed when gold dissolves in aqua-regia is
tetrachloridoaurate (III)
tetrachloridoaurate (I)
tetrachloridoaurate (II)
dichloridoaurate (III)
Which of the following will be able to show geometrical isomerism?
MA3B - Square planar
MA2B2 - tetrahedral
MABCD- saquare planar
MABCD- tetrahedral
The complex formed when Al2O3 is leached from bauxite using conc. NaOH solution is,
Na[Al(OH)4]
NaAl2O4
Na[Al(OH)3]
Na2AlO2
The number of isomers possible for the octahedral complex [CoCl2(en)(NH3)2]+ is
two
three
no isomer
four isomers
The complex ion having minimum magnitude of Δ° (CFSE) is
[Cr(CN)6]3-
[Co(CN3)6]3+
[Co(Cl)6]3+
[Cr(H2O)6]3+
As per IUPAC norms, the name of the complex [Co(en)2(ONO)Cl]Cl is
chlorido bis (ethane-1, 2-diamine) nitro-O-cobalt (III) chloride
chlorido bis (ethylenediamine) nitro-O-cobalt (III) chloride
chlorido di (ethylenediamine) nitrocobalt (III) chloride
chloro ethylenediaminenitr-O-cobalt (III) chloride
Square planar complex of the type MAXBL (where A, B,X and L) are unidenate ligands) shows following set of isomers.
two cis and one trans
two trans and one cis
two cis and two trans
three cis and one trans
A.
two cis and one trans
Square planar complex of the type MAxBxL shows two cis and one trans isomers.
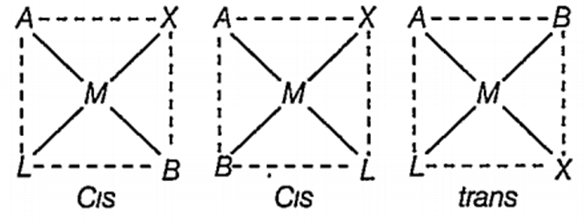
The formula of potash alum is :
K2SO4.Al2(SO4)3.24H2O
K2SO4.Al2(SO4)3.18H2O
K2SO4.(NH4)2SO4.18H2O
Na2SO4.Al2(SO4)3.24H2O
