 Multiple Choice Questions
Multiple Choice QuestionsGiven,Â
(i) Cu2+ + 2e- → Cu,   Eo = 0.337 V
(ii) Cu2+ +e- → Cu+, Eo = 0.153
Electrode potential, Eo for the reaction,
Cu +e- →Cu, will beÂ
0.52 V
0.90 V
0.30 V
0.30 V
The values of ΔH and ΔSfor the reaction, C(graphite) + CO2 → 2 CO (g) are 170 kJ and 170 JK-1 respectively. This reaction will be spontaneous atÂ
710 K
910 K
1110 K
1110 K
Kohlrausch's law states that at
finite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte, whatever be the nature of the other ion of the electrolyte.
infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ions of the electrolyte.
infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ions of the electrolyte.
Standard free energies of formation (in kJ/mol) at 298 K are -237.2, 394.4 and -8.2 for H2O (l), CO2 (g) and pentane (g), Â respectively. The value of Ecello for pentane-oxygen fuel cell is
1.968 V
2.0968 V
1.0968 V
1.0968 V
The equilibrium constant of the reaction:
Cu (s) + 2 Ag+ (aq) →  Cu2+ (aq) + 2 Ag (s);
Eo = 0.46 V at 298 K
2.4 x 1010
2.0 x 1010
4.0 x 1010
4.0 x 1010
D.
4.0 x 1010
Cu (s) + 2 Ag+ (aq) →  Cu2+ (aq) + 2 Ag (s)
Eo = 0.46 V at 298 K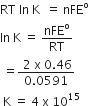
If   the standard emf of the reaction:
 the standard emf of the reaction:
Fe + 2 Fe3+ →3Fe2+
will be:
0.330 V
1.653 V
A hypothetical electrochemical cell is shown below
A- | A+ (xM)|| B+ (yM)|B+
The emf measured is +0.20 V. The cell reaction is:
A+ + B  →  A + B+
A+ + e-  →  A ; B+ + e-  → B-
the cell reaction cannot be predicted
the cell reaction cannot be predicted
Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.2 ×10–4 mol L–1. Solubility product
2.42 × 10–8
2.66 × 10–12
4.5 × 10–11
4.5 × 10–11
In the electrochemical cell :
Zn|ZnSO4(0.01M)||CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4Â is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is the relationship between E1 and E2?
(Given, RT/F= 0.059)
E1= E2
E1< E2
E1> E2
E1> E2
