 Multiple Choice Questions
Multiple Choice QuestionsThe solubility in water of a sparingly soluble salt AB2 is 1.0 × 10-5 mol L-1. Its solubility product number will be
4 × 10-15
4 × 10-10
1 × 10-15
1 × 10-10
The correct order of increasing basic nature for the bases NH3, CH3NH2 and (CH3)2NH is
CH3NH2 < NH3 < (CH3)2NH
(CH3)2NH < NH3 < CH3NH2
NH3 < CH3NH2 < (CH3)2NH
CH3NH2 < (CH3)2NH < NH3
The solubility of CaF2 in pure water is 2.3 ×10-6 mol dm-3. Its solubility product will be:
4.8 ×10-18
48.66 ×10-18
4.9 ×10-11
48.66 ×10-15
Two moles of PCl5 were heated in a closed vessel of 2 L. At equilibrium 40% of PCl5 is dissociated into PCl3 and Cl2.The,value of equilibrium constant is :
0.53
0.267
2.63
5.3
What is the equilibrium expression for the reaction
P4 (s) + 5O2 (g) P4O10 (s) ?
Kc =
Kc =
Kc = [O2]5
Kc =
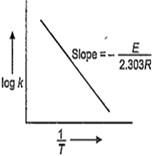
The temperature dependence of rate constant (k) of a chemical reaction is written in terms of Arrhenius equation, k = Ae-E*/ RT. Activation energy (E*) of the reaction can be calculated by plating
log k vs
log k vs
k vs T
k vs
A.
log k vs
Arrhenius equation k = Ae-E/ RT
ln k = ln A -
or, log k = log A -
Hence, E is calculated with the help of slope of following
The heats of combustion of carbon monoxide at constant pressure and at constant volume at 27°C will differ from one another by :
27 cal
54 cal
300 cal
600 cal
