 Multiple Choice Questions
Multiple Choice QuestionsThe decreasing order of acidic character among ethane (I), ethene (II), ethyne (III) and propyne (IV) is
(I) > (II) > (III) > (IV)
(II) > (III) > (I) > (IV)
(III) > (IV) > (II) > (I)
(IV) > (III) > (II) > (I)
The alkene that will give the same product with HBr in the absence as well as in the presence of peroxide is
2-butene
1-butene
propene
2-methylpropene
Chlorination of benzene in the presence of halogen carrier is an example of
aromatic nucleophilic substitution
aromatic electrophilic substitution
aromatic nucleophilic addition
aromatic electrophilic addition
Two organic compounds X and Y on analysis gave the same percentage composition namely; C = (12/13) × 100% and H = (1/13) × 100%. However, compound X decolourises bromine water while compound Y does not. The two compounds X and Y may be respectively
acetylene and ethylene
acetylene and benzene
ethylene and benzene
toluene and benzene
For preparing an alkane, a saturated solution of sodium or potassium salt of a carboxylic acid is subjected to
hydrolysis
electrolysis
oxidation
hydrogenation
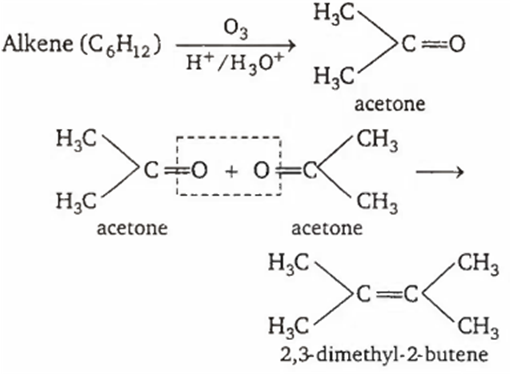
An organic compound with molecular formula C6H12 upon ozonolysis give only acetone as the product. The compound is
2,3-dimethyl-1-butene
3-hexane
2-hexene
2,3-dimethyl-2-butene
D.
2,3-dimethyl-2-butene
An aromatic hydrocarbon with empirical formula C5H4 on treatment with concentrated H2SO4 gave a monosulphonic acid. 0.104 g of the acid required 10 mL of NaOH for complete neutralisation. The molecular formula of hydrocarbon is
C5H4
C10H8
C15H12
C20H16
An alkene having the molecular formula C9H18 on ozonolysis gives 2, 2-dimethyl propanal and 2-butanone. The alkene is :
2, 2, 2-trimethyl-3-hexene
2, 2, 6-trimethyl-3-hexane
2, 3, 4-trimethyl-2-hexene
2, 2, 4-trimethyl-3-hexene
