 Multiple Choice Questions
Multiple Choice QuestionsObserve the following reactions and predict the nature of A and B:
A and B both are 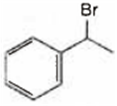
A and B both are ![]()
A is 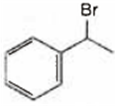 and B is
and B is ![]()
A is ![]() and B is
and B is 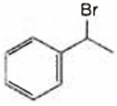
C.
A is  and B is
and B is ![]()
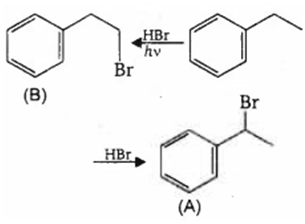
Nitration of aniline in strongly acidic medium, result in the formation of m-nitroaniline also. This is because :
amino group is meta orienting during electrophilic substitution reaction
nitro group goes always to the meta position irrespective of the substituents
nitration of aniline is a nucleophilic substitution reaction in strongly acidic medium
in strongly acidic conditions aniline is present as anilinium ion
Propyne when passed through a hot iron tube at 400°C produces :
benzene
methyl benzene
dimethyl benzene
trimethyl benzene
The name of the compound fig is:
![]()
(2Z, 4Z)-2, 4-hexadiene
(2Z, 4E)-2, 4-hexadiene
(2E, 4Z)-2, 4-hexadiene
( 4E, 4Z)-2, 4-hexadiene
The following reaction represent,
C12H26 → C6H12 + C6H14
substitution
synthesis
cracking
polymerization
Order of reactivity of C2H6, C2H4 and C2H2 is
C2H6 > C2H4 > C2H2
C2H2 > C2H6 > C2H4
C2H4 > C2H2 > C2H6
All are equally reactive.
In Wurtz reaction alkyl halide react with
sodium in ether
sodium in dry ether
sodium only
alkyl halide in ether
