 Multiple Choice Questions
Multiple Choice QuestionsThe carbon-carbon bond length in benzene is
in between C2H6 and C2H4
same as in C2H4
in between C2H6 and C2H2
in between C2H4 and C2H2
Which one of these is not true for benzene?
It forms only one type of monosubstituted product
There are three carbon-carbon single bonds and three carbon-carbon double bonds
The heat of hydrogenation of benzene is less than the theoretical value
The bond angle between the carbon-carbon bonds is 120°
Which one of the following conformations of cyclohexane is the least stable?
Half-chair
Boat
Twisted-boat
Chair
Which one of these is not true for benzene?
It forms only one type of monosubstituted product
There are three carbon-carbon single bonds and three carbon-carbon double bonds
Heat of hydrogenation of benzene is less than its theoretical value
The bond angle between carbon-carbon bonds is 120°
A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon. The derivative is
1, 1-dibromopropane
2, 2-dibromobutane
1, 2-dibromoethane
1, 4-dibromobutane
Which one of the following is an intermediate in the reaction of benzene with CH3Cl in the presence of anhydrous AlCl3?
Cl-
CH
CH
![]()
Cycloalkane formed when 1,4-dibromopentaneis heated with sodium is
methyl cyclobutane
cyclopentane
cyclobutane
methyl cyclopentane
Methane can be converted into ethane by the reactions
chlorination followed by the reaction with alcoholic KOH
chlorination followed by the reaction with aqueous KOH
chlorination followed by Wurtz reaction
chlorination followed by decarboxylation
Least energetic conformation of cyclohexane is
chair conformation
boat conformation
cis conformation
E-Z form
A.
chair conformation
Cyclohexane exists in two forms and these are chair and boat conformations.In the chair form, all the C-H bonds on adjacent carbon atoms, are in the skew position. In the boat form, four of the C-H bonds are skew (1,2,3,4, 4, 5 and 6, 1) and two are eclipsed (2, 3 and 5, 6). At the same time, there will also be some bond opposition strain for these two pairs of eclipsed bonds and also steric repulsion between the hydrogen pointing towards each other at 1 and 4.
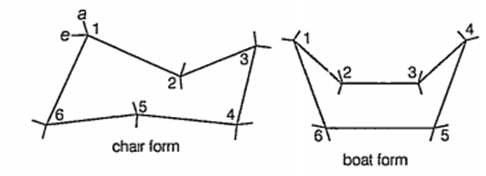
Hence, the total strain mn the boat conformation is larger than that in the chair conformation and consequently the former is less stable than the latter.
An alkyl bromide (X) reacts with sodium in ether to form 4,5-dIethyl octane, the compound X is
CH3(CH2)3Br
CH3(CH2)5Br
CH3(CH2)3CH(Br)CH3
CH3—(CH2)2—CH(Br)—CH2—CH3
