 Multiple Choice Questions
Multiple Choice QuestionsAt 80o C, the vapour pressure of pure liquid ‘A’ is 520 mm Hg and that of pure liquid ‘B’ is 1000 mm Hg. If a mixture solution of ‘A’ and ‘B’ boils at 80o C and 1 atm pressure, the amount of ‘A’ in the mixture is (1 atm = 760 mm Hg)
52 mol percent
34 mol percent
48 mol percent
48 mol percent
The vapour pressure of water at 20oC is 17.5 mm Hg. If 18 g of glucose (C6H12O6) is added to 178.2 g of water at 20oC, the vapour pressure of the resulting solution will be
17.675 mm Hg
15.750 mm Hg
16.500 mm Hg
16.500 mm Hg
D.
16.500 mm Hg
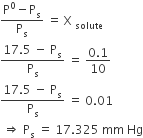
Equal masses of methane and oxygen are mixed in an empty container at 25ºC. The fraction of the total pressure exerted by oxygen is
2/3
(1/3) x (273 / 298)
1/3
1/3
A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol–1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 gcm–3, molar mass of the substance will be –
90.0g mol–1
115.0g mol–1
105.0g mol–1
105.0g mol–1
The density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29% H2SO4 (Molar mass = 98 g mol-) by mass will be
1.64
1.88
1.22
1.22
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be
350
300
360
360
Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is
1.14 mol kg–1
3.28 mol kg–1
2.28 mol kg–1
2.28 mol kg–1
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100o C is
759.00 Torr
7.60 Torr
76.00 Torr
76.00 Torr
The volume of a colloidal particle, VC as compared to the volume of a solute particle in a true solution VS, could be
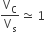
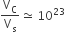
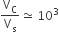
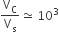
The solubility product of a salt having general formula MX2, in water is 4 x 10-12. The concentration of M2+ ions in the aqueous solution of the salt is
2.0 x 10-6 M
1.0 x 10-4 M
1.6 x 10-4 M
1.6 x 10-4 M
