 Multiple Choice Questions
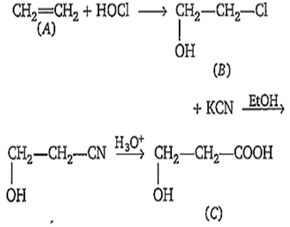
Multiple Choice QuestionsOne percent composition of an organic compound A is, carbon : 85.71% and hydrogen 14.29%. Its vapour density is 14. Consider the following reaction sequence-
Identify .
CH3CH(OH)-CO2H
HO-CH2-CH2-CO2H
HO-CH2-CO2H
CH3-CH2-CO2H
B.
HO-CH2-CH2-CO2H
C = 85.71% = = 7.14; = 1
H = 14.29% = = 14.29; = 2
Empirical formula = CH2
and empirical formula weight = 12 + 2 = 14;
Again,
Molecular formula weight = 2 × vapour density
= 2 × 14 = 28
n = = 2
Molecular formula = (CH2)2 = C2H4
At 25°C, the molar conductances at infinite dilution for the strong electrolytes NaOH, NaCl and BaCl2 are 248 × 10-4, 126 × 10-4 and 280 × 10-4 Sm2 mol-1 respectively, (BaOH)2 in Sm2 mol-1 is
52.4 × 10-4
524 × 10-4
402 × 10-4
262 × 10-4
The number of molecules of CO2 liberated by the complete combustion of 0.1 g atom of graphite in air is
3.01 × 1022
6.02 × 1023
6.02 × 1022
3.01 × 1023
19 g of a mixture containing NaHCO3 and Na2CO3 on complete heating liberated 1.12 L of CO2 at STP. The weight of the remaining solid was 15.9 g. What is the weight (in g) of Na2CO3 in the mixture before heating?
8.4
15.9
4.0
10.6
Solution 'X' contains Na2CO3 and NaHCO3, 20 mL of X when titrated using methyl orange indicator consumed 60 mL of 0.1 M HCl solution. In another experiment, 20 mL of X solution when titrated using phenolphthalein, consumed 20 mL of 0.1 M HCl solution. The concentrations (in mol L-1) of Na2CO3 and NaHCO3 in X are respectively
0.01, 0.02
0.1, 0.1
0.01, 0.01
0.1, 0.01
The number of moles of electrons required to deposit 36 g of Al from an aqueous solution of Al(NO3)3 is (At. wt. of Al = 27)
4
3
2
1
A carbon compound contains 12.8% of carbon, 21.% of hydrogen and 85.1% of bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula of the compound.
(Atomic weight : H = 1.008; C = 12.0; Br = 79.9)
CH3Br
CH2Br2I
C2H4Br2
C2H3Br3
3.011 × 1022 atoms of an element weight 1.15 gm. The atomic mass of the element is
23
10
16
35.5
A gas 'X' is dissolved in water at 2 bar pressure. Its mole fraction is 0.02 in solution. The mole fraction of water when the pressure of gas is doubled at the same temperature is
0.04
0.98
0.96
0.02
KMnO4 reacts with KI in basic medium to form I2 and MnO2. When 250ml of 0.1M KI solution is mixed with 250ml of 0.02M KMnO4 in basic medium, what is the number of moles of I2 formed?
0.015
0.0075
0.005
0.01
