Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following oxides of nitrogen dimerises into colourless is solid/ liquid on cooling?
N2O
NO
N2O3
NO2
Halogens exist in -1,+ 1, + 3, + 5 and 47 oxidation states. The halogen that exists only in -1 state is
F
Cl
Br
I
Which of the following is the correct method of preparation of methyl fluoride?
CH4 + HF →
CH3OH + HF →
CH4 + F2 →
CH3Br + AgF →
Which of the following species can function both as oxidising as well as reducing agent?
Cl-
ClO-
Which of the following is used as an oxidiser in rocket propellants?
Ammonium perchlorate
Alcohol
Acrylic rubber
Polyurethane
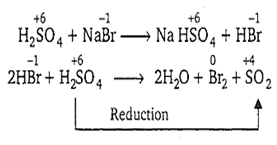
Concentrated sulphuric acid can be reduced by
NaCl
NaF
NaBr
NaOH
C.
NaBr
Concentrated sulphuric acid, being a strong acid, oxidises bromides and iodides but not chlorides and fluorides since, the later are more electronegative. Hence, among the given options, it can be reduced only by NaBr.